


15th
Jun 2021
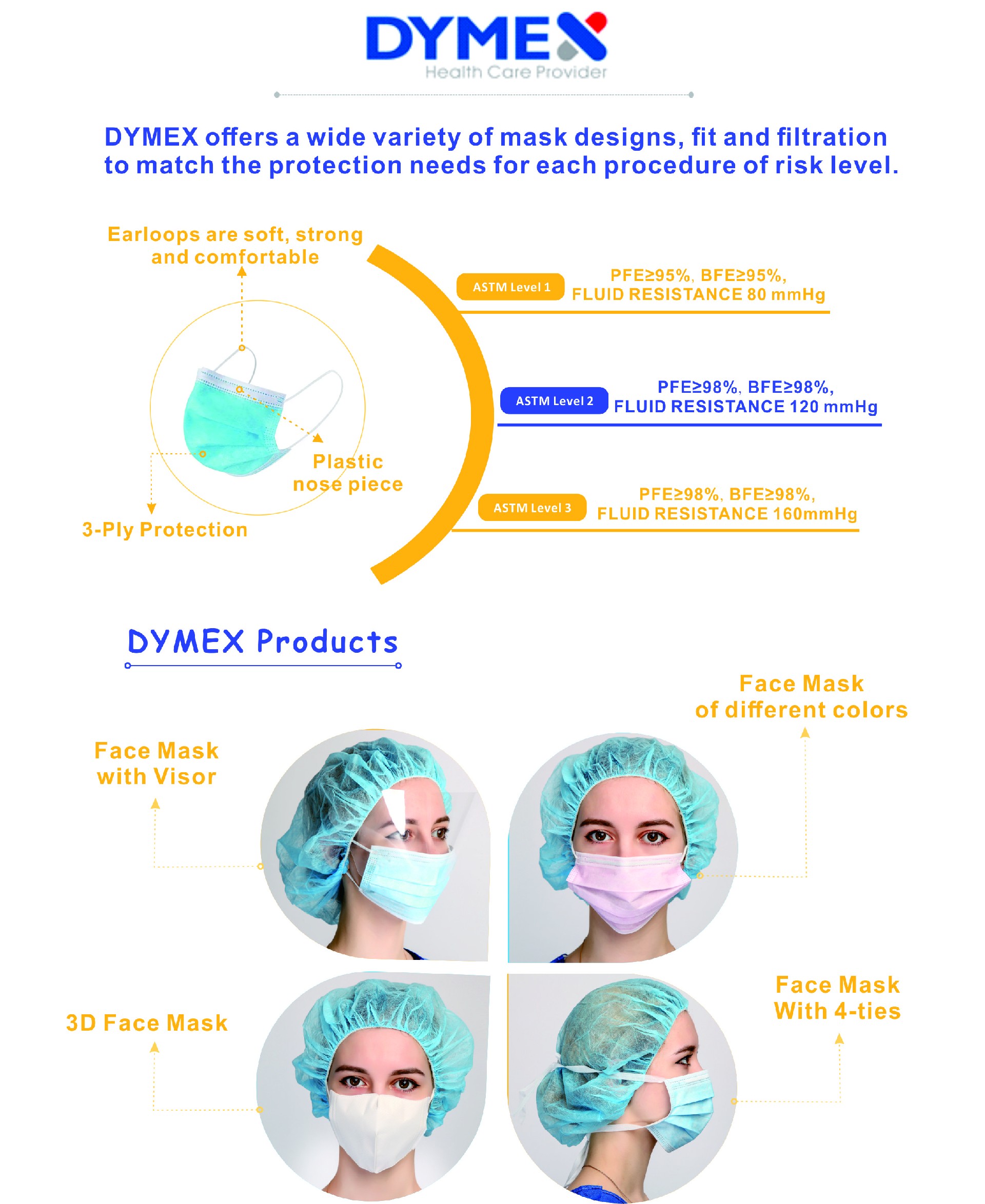
FDA has reviewed DYMEX’s 510(K) premarket notification of intent to market the device ( Surgical Face Mask ASTM Level 3 ) and have determined the device is substantially equivalent to legally marketed predicate devices marketed in interstate commerce prior to May 28, 1976, the enactment date of the Medical Device Amendments, or to devices that have been reclassified in accordance with the provisions of the Federal Food, Drug, and Cosmetic Act (Act) that do not require approval of a premarket approval application (PMA). DYMEX may, therefore, market the device, subject to the general controls provisions of the Act.
At this point, DYMEX has obtained the qualification to market Surgical Face Mask both ASTM Level 2 and ASTM Level 3 in the Unites States.
<<Previous page
Next page >>